Acids and Bases
Atoms can gain or lose electrons in order to form ions in a process called ionization (compounds formed in this way are called ionic compounds). When ionic compounds dissolve in water, their ions separate from one another in a process called dissociation. One interesting feature of water and many other covalent compounds is that they too can dissociate into ions. Unlike ionic compounds, such as sodium chloride, they are not ionized before they dissociate; they accomplish ionization and dissociation at the same time.
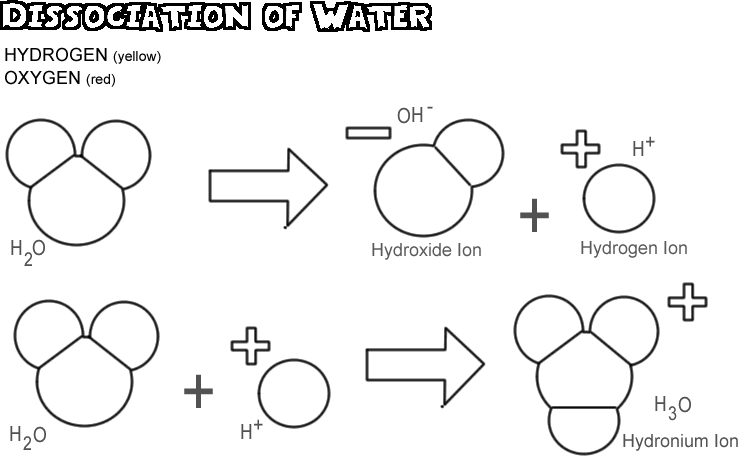
Dissociation of Water
When water dissociates, one of the hydrogen nuclei leaves its electron behind with the oxygen atom to become a hydrogen ion, while the oxygen and other hydrogen atoms become a hydroxide ion. Since the hydrogen ion has no electron to neutralize the positive charge on its proton, it has a full unit of positive charge and is symbolized as H+. The hydroxide ion retains the electron left behind and thus has a full unit of negative charge, symbolized by OH-. The hydrogen ion (proton) does not wander long by itself before it attaches to the oxygen atom of a second un-ionized water molecule to form a hydronium ion (H3O +)
In any sample of water, very few of the molecules are dissociated at any one time: in fact, only about one in 550 million. There is, however, a constant change; as one hydrogen ion reattaches to a hydroxide ion to form a water molecule, another water molecule dissociates to replace the hydrogen ion and the hydroxide ion in solution.
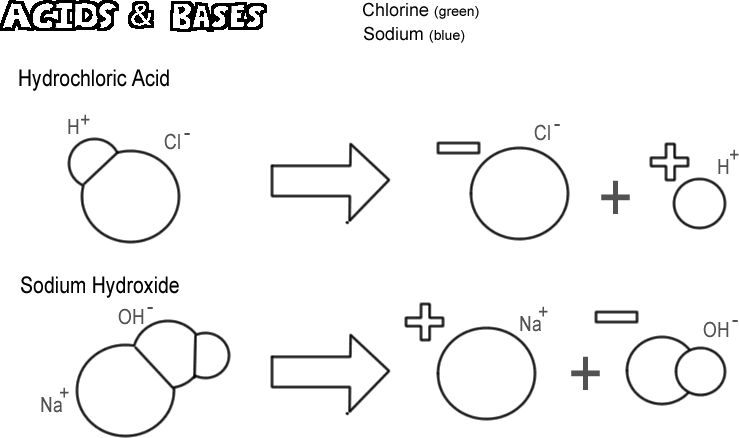
Hydrochloric Acid
Certain molecules, ionic and covalent, dissociate in such a way that they release a hydrogen ion without releasing a hydroxide ion. These substances are called acids. Since a hydrogen ion is really just a single proton in most cases, the chemist’s definition of an acid is a “proton donor.” If very many protons (hydrogen ions) are “donated” the effect can be very profound, such as burning your skin or dissolving metal. The acid illustrated is hydrochloric acid. Pure hydrochloric acid is a gas, but it dissolves easily in water to produce a solution of hydrogen ion and chloride ion. Since nearly all of it is dissociated in water, it is called a strong acid. Acids that do not dissociate completely are called weak acids.
Sodium Hydroxide
The opposite of an acid is a base, also known as an alkali. A typical strong base is sodium hydroxide, the principal component of lye. Sodium hydroxide dissociates to form a sodium ion and a hydroxide ion. A base is defined as a “proton acceptor.” The most common bases produce hydroxide ion when they dissociate, and it is the hydroxide ion that accepts the proton. A strong base can give your skin a much worse burn than an acid.
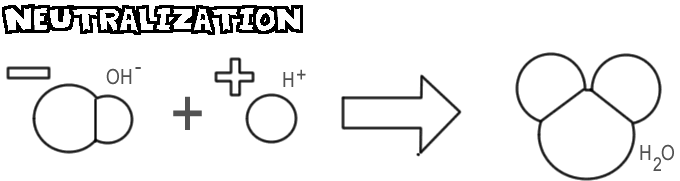
Neutralization
When a base and an acid are mixed, the hydroxide ion and the base combines with the hydrogen ion from the acid to form water. This process is called neutralization.
Questions:
1. What happens when an atom gains or loses an electron?
2. In your own words, explain why water generally has a neutral pH, even though water molecules dissociate.
3. Why are acids called proton donors?
4. What happens during neutralization?
5. Give an example of a strong base, and a strong acid.
