 Detecting the Duchenne Muscular Dystrophy (DMD) Mutation
Detecting the Duchenne Muscular Dystrophy (DMD) Mutation
PRELAB INFORMATION
Mary and John Smith have three children: Daniel, age 5; Alice, age 4; and Michael, age 1. Mary is two months pregnant. Recently Mary and John noticed that Daniel was having trouble climbing the stairs. He also complained several times that he was really tired after playing tag with his sister. Daniel’s doctor suggested some medical tests, which brought the family some bad news: Daniel has a disease called Duchenne muscular dystrophy (DMD).
Mary and John had never heard of DMD before, so they asked the doctor a lot of questions and went to the library for more information. They learned that DMD is a sex-linked genetic disease, which means that it results from damage to a gene on the X chromosome. That is why almost everyone with DMD is male. A girl may inherit an X chromosome with a defective copy of the DMD gene from her mother and like her mother; she will be a carrier for DMD. But the girl most likely will be protected from developing DMD by the normal X chromosome she gets from her father. In contrast, a boy does not get an X chromosome from his father, and if the X chromosome he gets from his mother carries a defective copy of the DMD gene, he will develop DMD.
Usually boys with DMD are healthy until the age of 4 or 5, at which time their muscles start to weaken. The doctor told the Smiths that Daniel would probably need a wheelchair in a few years, and that he would probably die before the age of 21. Although scientists are working to find a cure for this disease, there is no effective treatment for DMD now.
Obviously, Mary and John were very upset by this news about Daniel. Then they began to worry about their other children. Because Mary was a carrier for DMD, it was possible that Michael would develop DMD also, although he was too young to show any symptoms yet. They also worried about their unborn child and whether he or she might be at risk for DMD. Finally, even though Alice would not develop DMD, the Smiths wanted to find out whether she was a carrier like her mother. When the doctor explained that there is a genetic test that could determine whether each family member had the defective gene for DMD, Mary and John decided that they wanted this information. The family submitted the blood of each member for testing.
DNA was isolated from the white blood cells, but the DMD gene is only one of many thousands of genes. In order to study this particular gene, a procedure called PCR (polymerase chain reaction) is used to amplify the tiny section of DNA that contains the DMD gene. The techique uses DNA polymerase (the same enzyme that cells use to replicate their DNA before they divide) to replicate, over and over again, a particular DNA region of interest. With many copies of that gene in each tube, it becomes much easier to compare genes from different individuals and see if they are the same or different
Your job is to analyze the samples and determine which ones contain only copies of the wild-type DMD allele, which ones contain only copies of the mutant DMD allele, and which ones contain copies of both DMD alleles. As an expert, you know that the mutation that causes this disease involves a deletion of part of the DNA from the DMD gene, so that the mutant alleles will be shorter than the wild-type alleles. As illustrated in the diagram, this implies that Mary, who is a carrier for DMD, has one normal and one short (mutant) allele at the DMD locus. Since John, being a male, has only one X chromosome and does not have DMD, his DMD allele must be of normal length. Daniel has the DMD disorder, so he must have received the X chromosome with the defective DMD gene from his mother.
DNA molecules that differ in length can be separated and analyzed by a process known as agarose gel electrophoresis. This method uses an electric current to push DNA molecules through a gel-like substance called agarose. Small DNA molecules move through the gel faster than large ones.
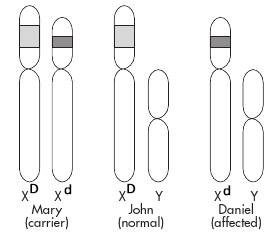
You will use agarose gel electrophoresis to simulate the procedure that the genetic counseling lab would use to determine the genotypes of a set of family members with respect to a gene of interest – such as the DMD gene. Each family member’s DNA sample would then be seen to contain one or both of two different sizes of DNA fragments: small or large. From this information, the counselor would then determine whether each individual had only the normal allele, only the mutant allele, or one of each (and therefore was a carrier).
Your job will be to determine which allele(s) each member of the Smith family possesses, by inspecting the gel at the end of the electrophoresis.
PRELAB QUESTIONS
1. What are the symptoms of Duchenne Muscular Dystrophy?
2. Illustrate using a Punnett square the method of inheritance showing Mary and John, and the four genotypes possible in their offspring.
3. What is the purpose of the PCR procedure?
4. Describe how the wild-type allele differs from the mutant DMD allele.
5. What is the relationship between the size of the DNA fragments and their appearance during gel electrophoresis?
Day 1: Setting the Gel
MATERIALS: For each group of students (group size to be determined by equipment availability):
- Gel-casting combs/trays and 50 ml of 0.8% agarose in water (1.6 g to 200 ml of water, boiled then cooled)
- A gel electrophoresis chamber and power supply
- 1 20 μl micropipettor
- 6 dye samples (one for each family member)and 6 pipette tips
PROCEDURE
1. Prepare the agarose gel:
a. Seal the ends of the gel-casting tray with masking tape. Insert the comb near one end of the casting tray. If your teacher instructs you to put two combs in your tray, so that two groups of students can run their samples at the same time, the second comb should be placed just beyond the center of the tray. Place the tray on a level surface where it will not be disturbed while the gel solidifies.
b. Carefully obtain a flask with melted agarose solution from the water bath or hot plate. It should be warm and liquified but not too hot to handle.
c. Carefully pour about 50 ml of the agarose solution into the casting tray, to fill it to a depth of about 4 mm. The gel should cover only about one-third of the height of the comb teeth.
d. Do not move or jar the casting tray while the agarose solidifies. As it becomes solid (10-15 minutes), the agarose will change from clear to cloudy.
e. When the agarose is solid, cover it with plastic wrap, leaving the comb and the tape intact. The casting tray will be refrigerated and will be used the next day.
Day 2 - Electrophoresis
2. Prepare the Chamber
a. Take the gel to the area where the gel electrophoresis chamber has been set up next to the power supply. Place the gel in the electrophoresis chamber so that the end containing the comb is toward the black electrode. Fill the chamber with tap water to a level that just covers the entire surface of the gel. Some chambers have "fill line" indicators on the plastic.
b. Slowly and gently remove the comb, taking care not to rip the gel. The water will lubricate the comb so it pulls out more easily.
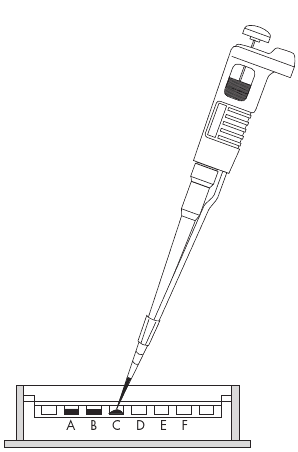 3. Load the samples:
3. Load the samples:
a. Use a micropipettor to load 5 μl of each sample into separate wells as shown in the diagram below. Leave one well empty at each end. Keep track of which sample was loaded into each well.
b. Using two hands, steady the micropipettor over a well.
c. Carefully dip the pipette tip through the surface of the water, center it over the well, and then gently depress the pipette plunger to slowly expel the sample into the appropriate well. If the tip is centered over the well, the DNA will sink to the bottom of the well. Do not release the plunger until the tip is out of the buffer.
3. To separate DNA through electrophoresis:
a. Avoid moving or bumping the electrophoresis chamber as you do the following: Close the top
of the electrophoresis chamber and connect the electrical leads to the power supply, red to red
and black to black. Make sure both electrodes are connected to one channel of the power supply. ![]()
b. Notify your teacher that you are ready to run your gel so she can check the setup. ![]() Instructor Initials __________
Instructor Initials __________
c. As soon as you get your teacher’s approval, set the power source to 130 volts (high) and turn
on the unit. ![]()
CAUTION: Electric shock hazard! Do not put fingers or other objects into the box while power supply is on.
e. Shortly after the current is applied, you should see the samples moving into the gel. ![]()
f. Run the electrophoresis for approximately 15 minutes. Check to monitor the progress of the
dye bands. (If you leave the gel running for too long, the dyes will run out the end of the gel and
get lost in the water.) ![]()
g. When the dyes have moved far enough to be distinguishable, turn off the power
supply, disconnect the leads. ![]()
h. You can remove the lid to the chamber now for a clearer view of the dye bands. ![]()
DMD DIAGNOSIS WORK SHEET
1. On the diagram below, a) above each well, put the letter that was on the sample that was loaded into that well and draw all the bands that you observed on your gel after electrophoresis.
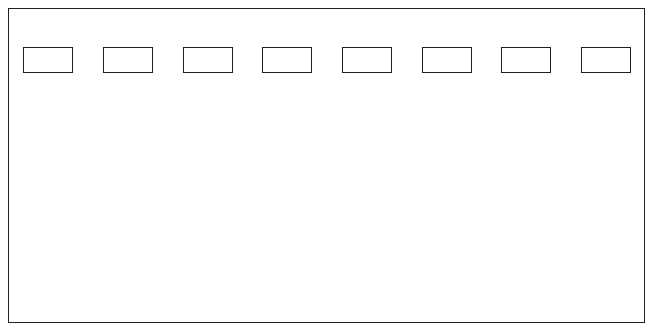
2. Indicate the genotypes and phenotypes for each of the family members
Mother Mary = X D X d , carrier | Father John = X D Y , normal
Daniel __________________________________
Alice __________________________________
Michael __________________________________
Fetus __________________________________
3. Which allele moves further into the gel, the normal (X D) or mutant (X d) allele? Why?
4. Why are most patients with DMD male? Explain how it would be possible or not possible for a female to have this disease.
5. Can a boy be a carrier for DMD without having the disease? Why or why not?
6. If you were the genetic counselor in this case, what would you tell the Smiths about their test results? Be specific with your diagnosis.
7. What would you expect the gel bands to look like if the Smiths had a daughter who was not a carrier? Defend your answer.
8. If you were Alice, would you choose to not have children. What if you were Michael? You must explain your choices with evidence-based reasoning from the project.
ADVANCE PREPARATION OF CHEMICALS
Materials Can be Obtained from:
Carolina Biological (800) 334-5551 --- www.carolina.com
Electrophoresis Power Supply
Gel Electrophoresis Chamber
Extra Comb-
Extra Gel Casting Tray
Agarose (powder)
Pipette Tips
Microcentrifuge tubes
Sigma Chemical Company (800) 325-3010 --- www.sigma/aldrich.com/order
Bromphenol Blue- catalog # B0126
Xylene Cyanole- catalog # X4126
You can also order "GEL LOADING SOLUTION" and Bromphenol Blue from Flinn Scientific
1. Prepare 0.8% agarose solution, 50 ml per group. The following recipe is for 200 ml(4 groups):
• To 200 ml water in a 500 ml flask add 1.6 grams agarose.
• Cap flask with foil and heat carefully on a hot plate, or cap with plastic wrap and heat carefully in a microwave oven for 3-5 minutes.
Safety Note: Agarose solution can superheat and either boil over during heating or erupt violently when the flask is touched. Handle hot agarose very carefully, wearing safety goggles and heavy gloves.
• Swirl flask and make sure that all agarose has dissolved.
• If gels are to be poured right away, cool flask to about 60°C before pouring.
• If gels are to be poured later the same day, hold flask in a 60°C water bath until use.
• If gels are to be poured another day, store solution covered and refrigerated. (Keeps forseveral weeks.) Then, well in advance of scheduled use, reheat agarose carefully on a hot plate or in a microwave oven until it is completely melted. Then bring it to about 60°C before pouring gels. (cool enough to touch to inside of wrist) If you will be pouring the gels yourself, follow the instructions given on the student pages for this exercise. When the gels have solidified, wrap each one in plastic wrap to prevent it from drying out.
2. Prepare stock solutions of bromphenol blue and xylene cyanole. In this exercise we will simulate the DNA samples of the various Smith family members with a pair of dyes, bromphenol blue and xylene cyanole. Bromphenol blue (BB) travels faster in an agarose gel, so it will be used to represent the "defective allele" that is a partially deleted version of the dystrophin (DMD) gene. Xylene cyanole (XC) travels more slowly in the gel, and so it will be used to represent the "normal" dystrophin allele.
Prepare stock solutions as follows:
Dye can be ordered from biology supply companies or from Amazon
• Label two small beakers or flasks BB and XC. Add 10 ml of deionized water and 1 ml
of glycerol to each. Swirl to mix. Safety Note: Certain dyes can be dangerous to your health when they are in the dry state
but become harmless once they have been dissolved. So weigh out all dyes in a fume
hood, and wear a dust mask, goggles, and gloves until they are in solution.
• Weigh out 0.025 grams (25 mg) of bromphenol blue and add it to the BB container.
• Weigh out 0.025 grams (25 mg) of xylene cyanole and add it to the XC container.
• Carefully stir or swirl both containers until the dyes are thoroughly dissolved.
3. Prepare simulated Smith family DNA samples from the stock dye solutions. Set up six microcentrifuge tubes, lettered A–F, for each group. Add dyes as shown in the table below. Then cap the tubes and place each set of six in a sandwich baggie or other small container to hand out to each lab group. As you begin the experiment, inform the students
which family member is represented by which letter, but do not provide the other information in the table.
Tube Family member Genetic condition Dye sample used
A Mother Carrier 25 μl BB & 25 μl XC
B Father Normal 50 μl BB
C Daniel Affected 50 μl XC
D Alice Carrier 25 μl BB & 25 μl XC
E Michael Normal 50 μl BB
F Fetus Carrier 25 μl BB & 25 μl XC
Adapted from: Modern Genetics for All Students
TIP: Many colleges will loan you materials, such as electrophoresis chambers, power supplies, and micropipettes
Related Documents: Article: Treating Genetic Disorders Using Exon Skipping | Worksheet: X-Linked Genes

